Sb can exist in a variety of oxidation states in nature but is most commonly found in either antimonite (Sb(III)) or antimonate (Sb(V)). Although trivalent Sb compounds are generally considered to be more toxic than pentavalent Sb compounds, the mobility of Sb(III) in the environment is significantly inferior compared to that of Sb(V). Therefore, it is of great significance to study the migration and transformation of trivalent and pentavalent Sb compounds in different natural water conditions for predicting their potential environmental risks. As previous studies indicated, Sb(V) and Sb(III) are thermodynamically stable in aerobic and anaerobic conditions. However, Sb(V) has been found in anoxic condition, and Sb(III) has also been detected in various oxygen-contained systems. These observations were explained by various abiotic and biological mechanisms. Compared to those abiotic hypotheses, the involvement of microorganisms in the biogeochemical cycle of Sb seems more reasonable. There are a great deal of studies that were carried out to illuminate the microbe-mediated transformation process and mechanism between Sb(V) and Sb(III). Compared to the detailed data on the microbial oxidation of Sb(III), the knowledge of the microbial reduction of Sb(V) is rather insufficient.
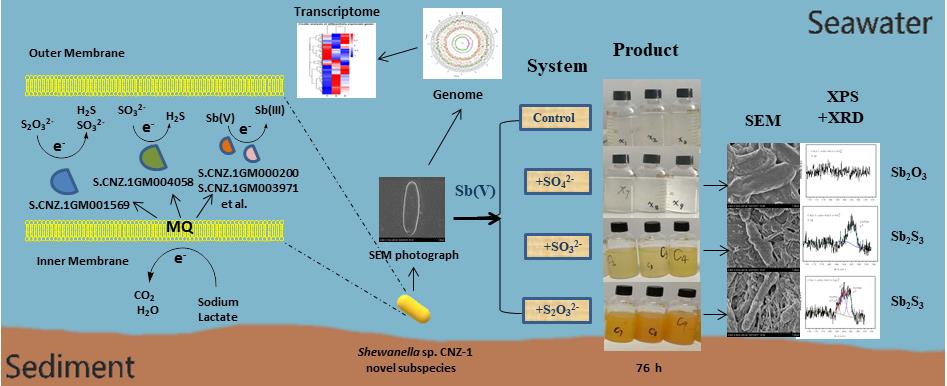
Based on the previous work, a research team from Yantai institute of coastal zone research (CAS) first reported that Sb(V) could be reduced by a marine bacterium Shewanella sp. CNZ-1 under anoxic and high-salt conditions. Moreover, the supplementation of SO32- and S2O32- into the reaction system can lead to the formation of Sb2S3 along with the reduction of SO32- and S2O32-, while SO42- can’t. Combined with the results of genome, transcriptome and RT-qPCR assays, the key genes that account for Sb(V) reduction by CNZ-1 in the presence of Sulfur-containing compounds were revealed, and the corresponding electron transfer path way was proposed (TOC).
These results were recently published in Chemical Engineering Journal (IF2018 = 8.335). Professor Xiaoke Hu and Dr. Haikun Zhang are the corresponding author and the first author of this paper. The present research was supported by the national natural science foundation of China (NSFC), the External Cooperation Program (CAS), the Key Research Project of Frontier Science (CAS) and other programs.
TOC
Link: https://www.sciencedirect.com/science/article/pii/S1385894718324021
https://aiche.onlinelibrary.wiley.com/doi/full/10.1002/btpr.2727
https://pubs.rsc.org/en/content/articlehtml/2017/ra/c7ra07438g